A new era of diabetes treatment
This content is independently produced by Australian Doctor Group with a grant from AstraZeneca.
Australian guidelines on the management of type 2 diabetes mellitus (T2DM) look set to change after a large body of international evidence confirmed a greater role for some of the newer anti-diabetic drugs in cardiovascular disease (CVD). Associate Professor John Amerena, who heads up cardiology research at Victoria’s Barwon Health, says Australian guidelines on T2DM management are poised for change. He predicts they will soon be updated to reflect the latest recommendations from Europe and the US where there is a renewed focus on cardiovascular (CV) risk management. Assoc Prof Amerena cites a recent report from the American College of Cardiology which recommends using sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) to cut the risk of major adverse cardiovascular events (MACE) in T2DM patients. The ADA/EASD consensus report makes similar recommendations, urging earlier prescribing of second-line therapies, GLP-1 RAs or SGLT2is, for patients with established atherosclerotic cardiovascular disease (ASCVD), heart failure and chronic kidney disease. The opportunity for CVD prevention in patients with T2DM has now expanded, Assoc Prof Amerena notes. “The guidelines from Europe in particular, are very clear recommending consideration of these agents earlier rather than later,” he says. “There is real benefit for the patients if you do so.” Metformin remains a first-line therapy for T2DM but clinical practice guidelines are starting to recommend an SGLT2i or a GLP-1 RA as second line therapy in patients with ASCVD. “I think there is plenty of evidence to suggest GPs should be giving strong consideration to these agents after metformin to lower glucose but also to improve cardiovascular outcomes,” he says. Assoc Prof Amerena cites results from several CV outcomes trials including the LEADER trial that found T2DM patients on the GLP-1 RA liraglutide had significantly reduced rates of MACE, along with the landmark EMPA-REG study that found patients taking the SGLT2i empagliflozin reduced MACE and were less likely to die than the placebo group. Even in those without established CVD, the latest evidence suggests some of the newer anti-diabetic agents play a more important role than previously thought. A large multinational real world observational study published in the JACC found the initiation of SGLT2is was associated with lower risk of death and heart failure (HF) regardless of pre-existing CVD. Similarly, results from the very recent DECLARE-TIMI 58 trial on CV outcomes using the SGLT2i dapagliflozin, found that patients with multiple risk factors for CVD but without established disease still got benefits with the results showing a significant reduction in hospitalisation for HF or CV death. This suggests a role for SGLT2is in both primary and secondary prevention of CVD. Assoc Prof Amerena notes that all the evidence from the CV outcomes trials shows that both SGLT2is “seem to have pretty much the same effect” but when it comes to deciding on which drug to use, he recommends GPs consider the evidence. As for the mechanisms responsible for the cardioprotective effects of SGLT2is and GLP-1 RAs, Assoc Prof Amerena says the jury is still out. “There are all these theories but nothing conclusive.” Here’s a snapshot of the updated EASD/ADA guidelines for second-line antidiabetic therapies for T2DM patients with established ASCVD: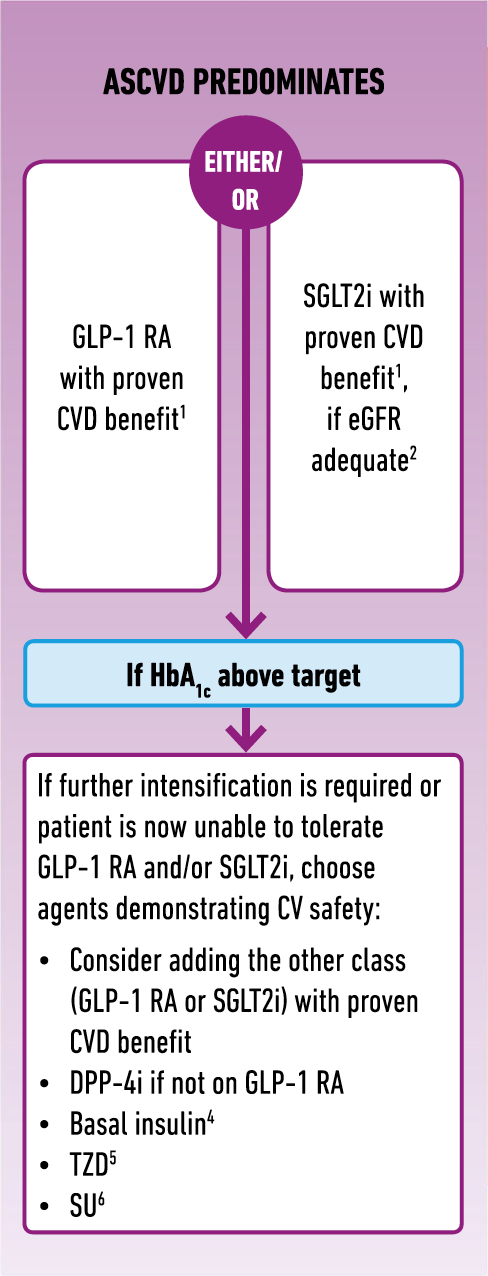
The full EASD/ADA guidelines can be viewed here.
Associate Professor John Amerena has received honorarium for speaking and consulting for Boehringer Ingelheim, Sanofi, Johnson & Johnson, Bayer, BMS, Amgen, Novartis, Pfizer, AstraZeneca and Servier.
